how to measure plant water potential
protocols for leaf water potential measurements
The following outlines a standard method for measuring leaf water potential. The method outlined in this page is suitable for many applications. However, Edaphic Scientific recommends you consider your application, project or situation to decide whether this method is most appropriate for you.
For assistance or more details, contact Edaphic Scientific.
cut the sample from the plant
 Preparing a sample for the pressure chamber usually involves removing a small lateral branch; if very small plants are used, the entire tip may be required. Removing the sample with a sharp knife or razor blade eliminates needless re-cutting, which can introduce considerable error in some species. The cut should be clean and normally at an angle makes for easier viewing.
Preparing a sample for the pressure chamber usually involves removing a small lateral branch; if very small plants are used, the entire tip may be required. Removing the sample with a sharp knife or razor blade eliminates needless re-cutting, which can introduce considerable error in some species. The cut should be clean and normally at an angle makes for easier viewing.
Because measurements should be taken as soon as possible after the sample is cut, it is advisable to take the instrument to the sampling site if possible. In many plants (especially new shoots and succulent plants) a delay in making a measurement may introduce significant error. For example, samples can be taken from dormant plants and stored for 20 minutes under cool, humid conditions before measuring with little error; however, succulent samples stored in warm, dry conditions for 20 minutes can experience an increase of 10 bar or more during that 20-
minute delay.
To avoid such errors, standardized measurement techniques based on sound physical and physiological principles must be used. As a general rule, a sample should be measured as soon as possible after being removed from the plant.
 If it is unavoidable to have a short time delay between excising the leaf from the plant, you should place the leaf in a reflective, sealable bag. This sealable bag will minimise the loss of water from the leaf for a short period of time.
If it is unavoidable to have a short time delay between excising the leaf from the plant, you should place the leaf in a reflective, sealable bag. This sealable bag will minimise the loss of water from the leaf for a short period of time.
seal the sample into the chamber lid
Next you will need to seal the sample into the chamber this can be done by using a Compression Gland Cover or by using gaskets, inserts insertion tools. Click on the one that applies to you and then return to this page to finish the tutorial on “Making the Measurement”. An erroneous measurement may result if a large proportion of a small, succulent sample (e.g. conifer needle, grass blade, new shoot, etc.) is squeezed by the gasket. For example, PMS can be successfully measured with a fascicle of pine needles, but substantial (5 to 10 bar) errors can occur in measuring needles from Douglas fir, even with a very thin gasket. So long as no more than 10% of the sample protrudes through or is squeezed by the gasket, this type of error should be negligible.
making the measurement
The control valve is turned to CHAMBER to increase the pressure inside the chamber. The cut surface of the plant sample which protrudes through the chamber cover is observed carefully. When water appears at the cut surface the control valve is turned to OFF immediately, and the pressure indicated on the gauge is recorded. The control valve is then turned to EXHAUST and when the chamber pressure reaches zero (indicated by the gauge), the chamber cover and sample can be removed. The instrument is ready for the next measurement.


The endpoint, or pressure at which water appears on the cut surface, is sometimes difficult to recognize. A bright light directed on the cut surface is probably the most important aid in recognizing the endpoint. A good quality hand lens (about 10 power) may also be useful, particularly for samples which are small in diameter.
When the endpoint is determined and nitrogen turned off with the control valve, there is a slight decrease in pressure even if there is no leak. This phenomenon is particularly noticeable at high stress levels with a rapid rate of pressure increase. It is caused by temperature changes inside the chamber and can be minimized by adjusting the rate of pressure increase.
Resin can obscure the endpoint and may be bothersome in certain species, particularly with pines. Resin forms bubbles which break and can be wiped away to allow observation of the true endpoint. Resin usually appears at very low pressures and need not interfere with accurate measurements. With experience, resin can be dealt with satisfactorily.
CAUTION!
Never place any part of your body directly over the chamber when the chamber is pressurized. It is possible that the sample may be forced out of the chamber at high velocity!
interpreting data
Decisions about plant water management usually cannot be made simply by measuring the PMS. However, PMS measurements can help us understand how the plant has responded to its environment and with experience, how we might modify that response. It is important to remember two points:
1. PMS changes in a regular pattern when the environment changes in a regular pattern. Although it is possible to infer past or future PMS levels from a single measurement, it is better to make a series of measurements to discover how the PMS is changing.
2. Knowing the PMS level is useful only if it can be related to a physiological or growth process of the particular plant. For example, an important level is the PMS at which photosynthesis begins to be inhibited, or the PMS level which results in a reduced yield per acre for a particular crop.
This kind of information is available from two sources:
- Published literature (World Wide Web or research papers)
- Research and experience.
Extrapolation of physiological response from one species to another is risky at best, but some generalizations can be helpful in getting started in the right direction. Following are some guidelines to interpreting PMS.
PREDAWN (or daily minimum) measurements of PMS range from 1.5 bar for plants in saturated soil and under low atmospheric demand to 60 or more bar for desert plants with almost no available soil moisture. The PMS of most plants just before dawn, however, will be between 3 and 10 bar. Plants having a PMS of 3 bar are probably not limited by soil water supply, even during the day, and probably would not respond to irrigation, Plants having a PMS of 10 bar are probably limited in certain physiological processes. These processes will become even more limited during the day as the PMS increases. As the PMS exceeds 10 bar (predawn) the plant becomes more severely limited until it shuts down and eventually will die if it does not receive water. The complexity of the interaction between the environment and plants emphasizes the need to measure the PMS not only at the predawn minimum, but also at times when the PMS is higher.
MAXIMUM PMS occurs during the day, usually when the solar radiation is maximum (early afternoon). A series of measurements taken during the day, is useful in determining when the maximum PMS occurs and when it should be measured (i.e., when the PMS is changing slowly). A maximum PMS of 7 bar does not limit the physiological processes of most plants. The degree to which PMS above that level limits plant functions depends upon the plant species. Recognizing that large variations occur from one species to another, a general rule may be formulated that as the PMS increases from 5 to 17 bar, plants become more and more limited in their ability to grow. This is the range in which photosynthesis, cell elongation, and phloem transport become reduced, sometimes abruptly. If a plant has a PMS above 20 bar it will usually wilt, as is the case with most herbaceous plants, or shut-down, as is common with woody plants. As PMS increases above 20 bar plant vigor declines. Some time before the PMS reaches 100 bar most plants die. The ability of plants to survive conditions under which their PMS exceeds 20 bar is highly variable between species.
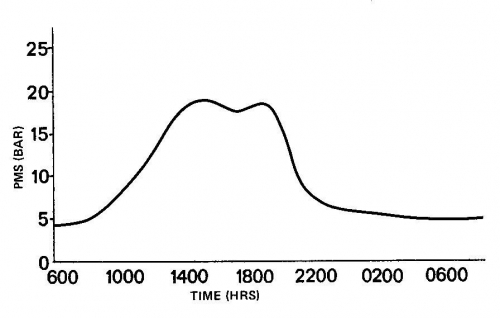
Change in PMS during the day for a plant with adequate water supply.
SEASONAL CHANGES are described by making weekly or monthly measurements. PMS may change even though there are no environmental changes because physiological and morphological changes can modify a plant’s response to its environment and hence change its PMS. Pressure/volume measurements (described in a subsequent section) can be made periodically to show seasonal changes in important physiological measurements, such as turgor pressure, osmotic concentration, and water potential of the plant.
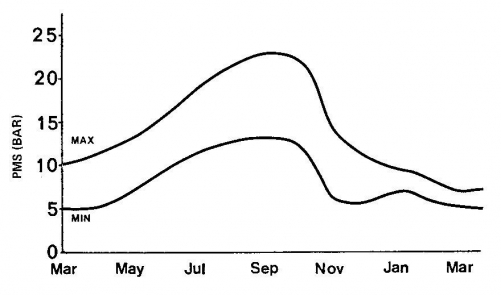
Annual change in Maximum and Minimum daily PMS.


