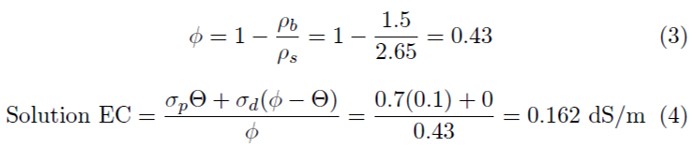soil salinity measurements explained....
Salinity is the amount of salts in soil. Soil salinity, or the saltiness of soil, specifically refers to the amount of sodium chloride (NaCl) concentration in the soil.
The scientific definition of salt, however, means ionic compounds that break down into ions when dissolved. Some example compounds that form salts include nitrate, nitrite, phosphate and potassium. However, in discussions of soil salinity, these compounds are ignored and the focus is on NaCl.
how to measure soil salinity
 The most practical and cost-effective method to measure soil salinity is via electrical conductivity (EC) sensors. The TEROS-12 is an example of an EC sensor, which can also measure soil volumetric water content and temperature, that can either be connected to an ZL6 or ES-LOG for continuous data logging, the LoRaWAN or modem for remote telemetry monitoring.
The most practical and cost-effective method to measure soil salinity is via electrical conductivity (EC) sensors. The TEROS-12 is an example of an EC sensor, which can also measure soil volumetric water content and temperature, that can either be connected to an ZL6 or ES-LOG for continuous data logging, the LoRaWAN or modem for remote telemetry monitoring.
Electrical conductivity (EC) is the ability of a substance to conduct electricity and can be used to infer the amount of charged molecules that are in solution. EC is measured by applying an alternating electrical current to two electrodes, and measuring the resistance between them.
Conductivity (referred to as \bulk electrical conductivity") is derived by multiplying the inverse of the resistance (conductance) by the cell constant (the ratio of the distance between the electrodes to their area). TEROS-12 bulk EC measurements are normalized to EC at 25 °C. We factory calibrate the bulk EC measurement to be accurate within ±10% from 0 to 10 dS/m. This range is adequate for most greenhouse and nursery applications.
However, some special applications in highly saline substrates may require measurements with bulk EC greater than the speci ed range. The TEROS-12 will measure up to 23 dS/m bulk EC, but user calibration is required above 10 dS/m.
bulk EC versus pore EC versus solution EC
 When measuring EC in soils, it is important to know which type of EC is being measured. The interpretation and importance of the data will rely on which EC is being measured. In soils, there are three types of EC: bulk, pore water and soil solution EC.
When measuring EC in soils, it is important to know which type of EC is being measured. The interpretation and importance of the data will rely on which EC is being measured. In soils, there are three types of EC: bulk, pore water and soil solution EC.
Remember that the definition of EC here means the combination of all salts in the soil including NaCl and nutrients such as nitrogen phosphorous and potassium.
Bulk EC is the total amount of EC in the soil including dissolved in the solution and solid particles.
Pore water EC is the amount of EC, or salts, only in the pore space of soil. Pore water EC is also known as plant available EC and is the EC value most relevant to plants.
Solution EC is the EC of pore water removed from a saturated paste.
How to measure bulk, pore water and solution EC is outlined in more detail below.
converting bulk EC (σb) to pore EC (σp)
For many applications, it is advantageous to know the electrical conductivity of the solution contained in the soil pores (σp), which is a good indicator of the solute concentration in the soil. Traditionally, σp has been obtained by extracting pore water from the soil and measuring σp directly. As one would expect, this is a time consuming and labor intensive process.
The TEROS-12 measures the electrical conductivity of the bulk soil surrounding the sensors (σb). A considerable amount of research has been conducted to determine the relationship between σb and σp. Work by Hilhorst (2000), has taken advantage of the linear relationship between the soil bulk dielectric permittivity (εb) and σb to allow accurate conversion from σb to σp if the εb is known. The TEROS-12 measures εb and σb nearly simultaneously in the same soil volume. It is therefore well suited to this method.
The pore water conductivity can be determined from (see Hilhorst, 2000 for derivation):
σp= (εp σb) / (εb − εσb=0) (eq. 1)
where σp is the pore water electrical conductivity (dS/m); εp is the real portion of the dielectric permittivity of the soil pore water (unitless); σb is the bulk electrical conductivity, (dS/m), which is measured directly by the TEROS-12; εb is the real portion of the dielectric permittivity of the bulk soil (unitless); εσb=0 is the real portion of the dielectric permittivity of the soil when bulk electrical conductivity is 0 (unitless). ε can be calculated from soil temperature using:
εp = 80.3 − 0.37 × (Tsoil − 20) (eq. 2)
where Tsoil is the soil temperature (°C) measured by the TEROS-12.
Finally, εσb=0 is an offset term loosely representing the dielectric permittivity of the dry soil. Hilhorst (2000) recommended that εσb=0 =4.1 be used as a generic offset. However, our research in several agricultural soils, organic, and inorganic growth media indicates that εσb=0 = 6 results in more accurate determinations of σp. Hilhorst (2000) offers a simple and easy method for determining for individual soil types, which will improve the accuracy of the calculation of σp in most cases.
Our testing indicates that the above method for calculating σp results in good accuracy (± 20%) in moist soils and other growth media. In dry soils where VWC is less than about 0.10 m3/m3, the denominator of equation 1 becomes very small, leading to large potential errors. We recommend that p not be calculated in soils with VWC < 0.10 m3/m3 using this method.
pore water EC (σp) versus soil solution EC
As noted in the previous section, pore water electrical conductivity can be calculated from bulk EC using the sensor-measured dielectric permittivity of the medium. However, pore water EC is not the same as solution EC. Pore water EC is the electrical conductivity of the water in the pore space of the soil.
One could measure this directly if the soil was squeezed under high pressure to force water out of the soil matrix and that water was collected and tested for EC. Solution EC is the electrical conductivity of pore water removed from a saturated paste. In this case, wet the soil with distilled water until the soil saturates, then place the soil on lter paper in a vacuum funnel and apply suction. An electrical conductivity measurement on the water removed from the sample will give the solution electrical conductivity.
Theoretically, the two are related by the bulk density. An example calculation will illustrate this relationship: A soil is at 0.1 m3/m3 VWC, has a pore water EC of 0.7 dS/m, and a bulk density of 1.5 mg/m3.
We can calculate the solution EC as follows.
In this example, Φ is the porosity, ρb is bulk density, ρs density of the minerals (assumed to be 2.65 Mg/m3), subscript d is distilled water, and Θ is volumetric water content. We assume that the EC of the distilled water is 0 dS/m. In practice, solution EC calculated from this method and solution EC taken from a laboratory soil test may not agree well because wetting soil to a saturated paste is very imprecise.
measuring soil solution EC and NaCl directly
 In some cases, scientific researchers will want to measure soil solution EC directly rather than estimating from bulk or pore water EC. Some scientists may also want to know the specific levels of NaCl in the soil, rather than the combined total of all salt ions.
In some cases, scientific researchers will want to measure soil solution EC directly rather than estimating from bulk or pore water EC. Some scientists may also want to know the specific levels of NaCl in the soil, rather than the combined total of all salt ions.
The best method is to install rhizon samplers, also known as pore water samplers, sampling lysimeters, or suction lysimeters, into various depths of the soil profile. The rhizon samplers are also ideal for soil columns experiments in the lab.
Solution is collected in the rhizon samplers over a certain period of time. Once enough solution has been collected, the sample is then taken to the lab for EC measurements. In the lab, the TEROS-12 sensor can measure EC (if there is sufficient quantity of solution) or other lab based techniques can be used.
why we should measure salinity
 Soil salinity becomes a problem when the accumulation of salts (NaCl) affects plant growth.
Soil salinity becomes a problem when the accumulation of salts (NaCl) affects plant growth.
Small concentrations of salt may affect economically important crop and horticultural species, but many native Australian plants have adaptations to cope with relatively high soil salt concentrations.
Areas impacted by severe salt are "scalded", exposing bare soil to erosion and further land degradation. Salt scalds can be a prominent feature of the landscape where they are found.
Around 5.7 million hectares of land in Australia have been affected by salinity and it is estimated that this area will grow to over 17 million hectares by 2050. The global annual cost of land degradation caused by salinity is estimated at $US 27 billion.
Scientific researchers, environmental managers, and growers will have different reasons to measure and monitor salinity.
 Scientific researchers are broadly interested in salinity from the theoretical to the applied. Salinity poses many challenges to plant growth and reproduction. How plants evolved to cope with salinity is of intrinsic interest to plant physiologists and botanists. Some of these adaptations can be transferred to economically important crops to improve production in marginal lands. For example, recent advances in CRISPR-cas9 gene editing techniques may improve crop production by transferring genes from salt tolerant plants to crops such as wheat and rice.
Scientific researchers are broadly interested in salinity from the theoretical to the applied. Salinity poses many challenges to plant growth and reproduction. How plants evolved to cope with salinity is of intrinsic interest to plant physiologists and botanists. Some of these adaptations can be transferred to economically important crops to improve production in marginal lands. For example, recent advances in CRISPR-cas9 gene editing techniques may improve crop production by transferring genes from salt tolerant plants to crops such as wheat and rice.
Scientists work closely with environmental managers to manage the impact of salinity on the landscape. Science informs decisions that environmental managers need to make in order to prevent or alleviate land degradation caused by salinity. For instance, scientists have investigated the use plant growth-promoting bacteria (PGPB) to alleviate the toxic affects of salinity on plants. Particular success has been found when plants are inoculated with PGPB in combination with plant-symbiotic fungi (arbuscular mycorrhizal). Environmental managers can use such advances in plant-microbe interactions to alleviate salinity caused land degradation.
Growers have an obvious vested interest in monitoring and managing salinity on their land to prevent loss of crops and productivity. However, growers can also use salinity measurements to their advantage in soil nutrient and fertility management. It has been demonstrated that the accumulation of salts in the root zone indicates an accumulation of excessive nutrients (and NaCl) that may be detrimental to plant growth. Once salt has accumulated to a certain level, growers can "flush" their soil profile of excessive nutrients. This is one of many instances where salt measurements can be used by growers in crop production.
Conservationists are also concerned about the impacts of salinity on the landscape, biodiversity, ecosystem processes, and functional ecosystems. Highly degraded, saline land has an obvious impact on plant species and the types of species that can grow. Saline land may be highly toxic that prevents any plant growth; or one or a few halophytes (a salt tolerant plant species) may dominate the landscape.
the soil salinity problem in australia