hydrogen sulfide (H2S) sensors & meters....
 The AMT range of dissolved hydrogen sulfide (H2S) sensors are German designed and engineered for rapid and accurate measurements.
The AMT range of dissolved hydrogen sulfide (H2S) sensors are German designed and engineered for rapid and accurate measurements.
The dissolved H2S sensors are based on amperometric measurements. That is, the detection of H2S ions in a solution is based on electric current or changes in electric current. Also, the technology is referred to as a microsensor because the measurement tip is only 25 μm diameter. This allows for the rapid, in situ detection of hydrogen sulfide.
how does the sensor work?
The dissolved hydrogen sulfide sensor measures H2S based on partial pressure differences between the solution and the internal sensor's electrolyte.
That is, because of the partial pressure of the gaseous H2S, the analyte in the sensor is separated by permeation through an ion selective membrane. Inside the sensor, the hydrogen sulfide reacts with a redox mediator. The reoxidation at the working electrode causes an electical current corresponding to the concentration of the dissolved molecular H2S amount.
Furthermore, the sensor has a very short response time of approximately 200 milliseconds. Therefore, rapid measurements and sediment profiling with high resolution is possible.
minimal interference from ions and salts
Additionally, the ion selective membrane is highly discriminatory to H2S ions. Consequently, there are no interferences to analytes including CO, CO2, H2O-vapour, CH4 or NH3.
Also, salt concentrations of up to 40 g/l and turbid or coloured solutions do not interfere with the signal. Therefore, the dissolved hydrogen sulfide sensor can easily measure in extremely dirty solutions.
a range of measurement depths
The sensor and meter can measure dissolved hydrogen sulfide at various depths. The laboratory model (ES-H2S-L) is rated to 1 m depth and is ideal for lab or similar applications. The shallow water model (ES-H2S-SW) is rated to 100 m depth and is ideal for applications including marine environments, estuaries, lakes, rivers, waste water treatment plants, and more.
the advantages of microsensor technology
The Amerigo Lander is an example of a scientific instrument which has been designed to support dissolved hydrogen sulfide sensors.
The AMT dissolved H2S sensor is a microsensor because it has a special and unique geometric design.
These features include an electrode diameter less than 25 μm, a very thin ion selective membrane with small diameters, and extremely short diffusion distances between the solution and electrodes.
Consequently, the AMT dissolved hydrogen sulfide sensor has the following advantages:
- rapid response: the technology has a T90 response time of approximately 200 milliseconds;
- negligible analyte consumption for long-term measurements with minimal sensor replacement;
- low streaming and stirring sensitivity;
- in situ measurements at the micro scale; and
- ideal for applications such as biofilms and sediments.
how to measure total sulfides....
The AMT dissolved hydrogen sulfide sensor can measure total sulfides when measurements are combined with pH and temperature readings.
It is important to recognise that the sensor does not measure total sulfides directly. Rather, total sulfides is calculated from a combination of H2S, pH and temperature measurements.
H2S concentration is dependent on pH. Below a pH of ~4 a solution contains H2S. Between a pH of ~4 and ~8.5, a solution contains a combination of H2S and HS- (bisulfide or hydrosulfide). At pH values greater than 8.5, very little H2S remains (<2 % of the total sulfide amount) and the solution consists of HS- and increasing levels of sulfide (S2-).
This relationship between pH and the sulfide constituents are outlined in the following figure:
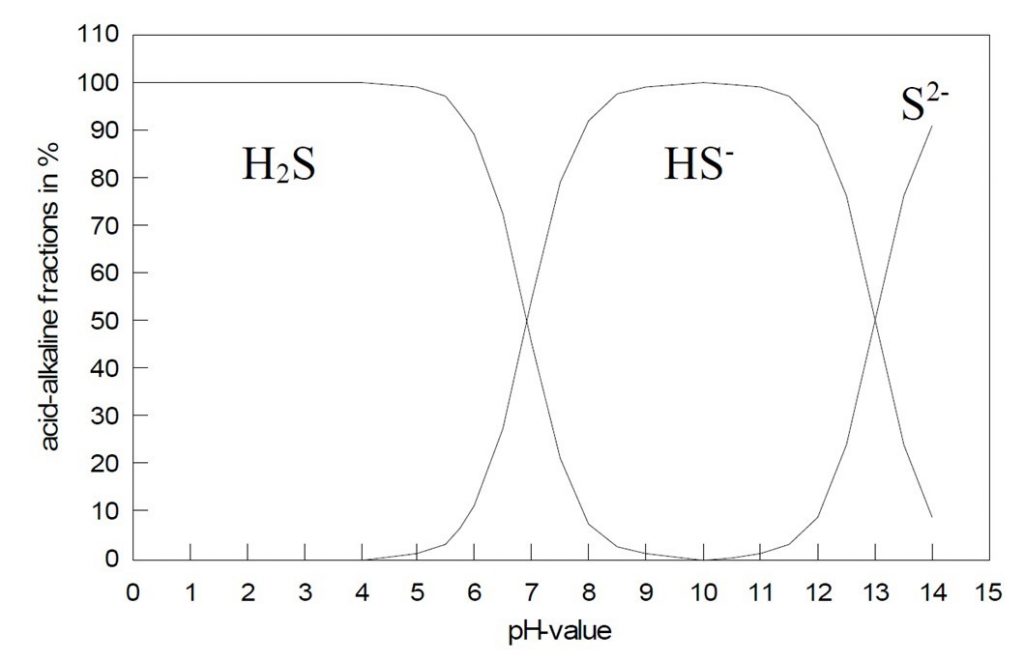
The relationship between hydrogen sulfide and pH. Acid-alkaline fractions depending on the pH. Image source: AMT GmbH.
Consequently, the calculation of the total sulfide amount (total sulfide amount = sum of the dissolved H2S in the sample + hydrogen sulfide + sulfide) is possible very easy by means of the pH dependence of the acid-alkaline fractions. Therefore, only one measurement is necessary for the determination of the H2S and of the total sulfide amount.
further information
- Hydrogen sulfide - a scientific review
- Hydrogen sulfide explained
- The Amerigo Lander: a novel underwater device to measure human impacts
a video on electrochemical reactions



