dissolved oxygen explained
what is dissolved oxygen?
Dissolved oxygen (DO) is the amount of oxygen present in a body of liquid solution, usually referring to freshwater or seawater.
“Normal” levels of dissolved oxygen can range between 5 and 14 mg/L. Readings above 18 mg/L are physically impossible.
A fully saturated freshwater sample will have a dissolved oxygen value of 8.26 mg/L at 25C; whereas a fully saturated seawater sample will have a dissolved oxygen value of 6.27 mg/L at 25C. The difference is due to the presence of salts, or salinity, in seawater and this is discussed in more detail below.
Watch a You Tube Video on Dissolved Oxygen
why is dissolved oxygen important?
 Dissolved oxygen is critical for life in water. Plant and animal organisms need oxygen for respiration. When dissolved oxygen levels fall below 5 mg/L, there is not enough oxygen in the water column for organisms to breathe. When dissolved oxygen remains below 5 mg/L for extended periods, it is possible for mass fish kills and other mortality events to occur.
Dissolved oxygen is critical for life in water. Plant and animal organisms need oxygen for respiration. When dissolved oxygen levels fall below 5 mg/L, there is not enough oxygen in the water column for organisms to breathe. When dissolved oxygen remains below 5 mg/L for extended periods, it is possible for mass fish kills and other mortality events to occur.
Productive aquaculture operations depend on optimal levels of dissolved oxygen. The growth rates and biomass accumulation in economic important species, such as salmon, require dissolved oxygen to be at certain levels throughout their life history. For example, mean dissolved oxygen for adults is around 6 mg/L, levels below 11 mg/L will affect growth and survival rates of eggs, and values less than 6 mg/L of dissolved oxygen will lead to the death of eggs (Carter, 2005). Therefore, aquaculture growers need to consider appropriate dissolved oxygen levels through manipulating aeration and stocking densities in order to maintain optimal dissolved oxygen levels.
Dissolved oxygen is also a general indicator of water quality for the wastewater industry. If nutrient levels or temperature are too high, then can be a large Biological Oxygen Demand (BOD) leading to increase respiration demand particularly by micro-organisms. Excessive nutrient loading or increase temperature can lead to a rapid increase in growth of micro-organisms that, in turn, can rapidly deplete dissolved oxygen levels in water. This can then effect downstream organisms and processes.
The toxicity of other elements in water can also change with varying levels of dissolved oxygen. For example, toxins such as lead, zinc and copper can double when dissolved oxygen levels fall from 10 mg/L to 5 mg/L.
what can change dissolved oxygen?
 Dissolved oxygen can vary due to a number of factors. Dissolved oxygen is a balance between oxygen producers and oxygen consumers in the water.
Dissolved oxygen can vary due to a number of factors. Dissolved oxygen is a balance between oxygen producers and oxygen consumers in the water.
Examples of oxygen producers include underwater plants, such as seagrass, or corals undertaking photosynthesis. Examples of oxygen consumers include micro-organisms, such as bacteria, or larger animals such as fish, crabs, lobsters, sharks and whales.
The atmosphere is another important source of oxygen in water bodies. Oxygen from the atmosphere diffuses into water (aeration) and from water (degassing) through Henry’s Law. This gas law states that “At a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.”. Therefore, a body of water will always come into equilibrium with the oxygen concentration of the atmosphere.
Watch a You Tube video explaining Henry's Law:
The level of dissolved oxygen in a water column, however, is rarely uniform and in equilibrium with the atmosphere. A major reason for this is the constant production of oxygen by photosynthesis as well as the consumption of oxygen by animals. There is still a strong stratification of dissolved oxygen in a water column and, without a significant stirring element, there is a long time lag for equilibrium in dissolved oxygen levels across the entire water column to be reached. This is because the diffusion of oxygen in water is extremely slow. The rate of diffusion of oxygen in water is about 10,000 times slower than in the atmosphere. As an example, in still water it can take 6 years for a dissolved oxygen molecule to diffuse from the surface to a depth of 6m.
physical variables, especially temperature, salinity and barometric pressure, can also affect dissolved oxygen levels
Temperature – The solubility of oxygen in water is affected by temperature. As temperature increases, oxygen becomes less soluble. The relationship between temperature and dissolved oxygen solubility is directly proportional and can therefore be easily predicted if temperature is known. For example, at 15 C water can dissolve 10.08 mg/L while 30C water can only dissolve 7.56 mg/L of oxygen.
Temperature also affects the molecular activity of oxygen and this, in turn, can alter the diffusion rate across a sensor’s membrane. As temperature decreases, molecular activity and diffusion rate also decreases and the measured readings can be lower than the actual dissolved oxygen level. If temperature is known, advanced sensors, such as the OPTOD Dissolved Oxygen Sensor, can automatically compensate for differences in temperature.
Salinity and electrical conductivity – As salinity, or electrical conductivity, increases in water, the solubility of oxygen decreases. The solubility of oxygen in sea water is about 20% less than it is in fresh water. This is because the salts in seawater occupy space that is no longer available to oxygen.
Salts can be any number of ions therefore it is more appropriate to measure electrical conductivity (EC) which is a measure of total ionic activity in a solution. Other ions may include nutrients such as phosphorus or nitrogen.
It is important that salinity, or, more precisely, electrical conductivity, is also measured when dissolved oxygen is measured – particularly in estuaries, tidal rivers, or marine environments.
Barometric pressure – In the Earth’s atmosphere, the concentration of oxygen is always 20.9%. However, the pressure of oxygen changes with barometric pressure. With a decrease in barometric pressure, there is a decrease in the partial pressure of oxygen pressure and the amount of oxygen that can be dissolved.
how is dissolved oxygen measured?
- Electrochemical or Clark-type sensors
- Optical sensors
- Photometers
- Oxygen microsensors
- Digital dissolved oxygen sensor
- Deep sea dissolved oxygen sensor
optical or electrochemical dissolved oxygen sensor?
Researchers, consultants, engineers and students need to consider whether an optical or electrochemical sensor is more suitable for their application. Both types of technology are excellent at measuring dissolved oxygen, however they also have their advantages and limitations.
This brief overview of electrochemical versus optical sensors mainly applies to macro sensors that maybe used in the wastewater or aquaculture industries. That is, this comparison does not necessarily apply to the oxygen microsensors and micro-optodes which have their own unique advantages and limitations that are discussed elsewhere.
Upfront versus ongoing costs – Electrochemical sensors tend to be cheaper to purchase upfront than optical sensors. However, electrochemical sensors need more ongoing maintenance, checking and calibration than optical sensors. These ongoing costs can be significant and need to be considered when purchasing dissolved oxygen sensors.
Accuracy – both electrochemical and optical sensors need to have a calibration in order to be accurate – particularly if salinity and temperature is a significant variation (see above for more details). A study by Leivouri et al (2014) compared 18 different types of dissolved oxygen sensors including optical and electrochemical sensors. They found that the optical sensors were generally more accurate and reliable than the electrochemical sensors. The major limitation for the electrochemical sensors, and why they performed poorly compared with optical sensors, was because they needed stirring or water movement around the sensor for accurate measurements.
Leivouri et al (2014) also found that the OPTOD Dissolved Oxygen Digital Optical Sensor was one of the most accurate of all of the dissolved oxygen sensors they compared.
Leivuori et al (2014) Field measurement intercomparison of dissolved oxygen sensor
measuring dissolved oxygen for different applications
There are a large range of sensors, meters and data loggers available to measure dissolved oxygen. There is not a single sensor or instrument that is suitable for all applications. Rather, sensors are designed for specific applications.
Edaphic Scientific recommends the continuous monitoring of dissolved oxygen rather than spot measurements. Continuous monitoring is able to capture all the diurnal and nocturnal variations in dissolved oxygen. Spot measurements may overestimate, or underestimate, the true dynamics of dissolved oxygen variations.
Below is a list of some applications and suggestions for how to measure dissolved oxygen.
 Wastewater Industry - Portable DO meter is ideal for spot checks and periodic measurements; a digital DO sensor can be connected to a SCADA or telemetry data logging system for continuous measurements, alerts and alarms. A photometer can also be used to measure dissolved oxygen in the wastewater industry. However, using a photometer can be time consuming relative to an optical or electro-chemical sensor.
Wastewater Industry - Portable DO meter is ideal for spot checks and periodic measurements; a digital DO sensor can be connected to a SCADA or telemetry data logging system for continuous measurements, alerts and alarms. A photometer can also be used to measure dissolved oxygen in the wastewater industry. However, using a photometer can be time consuming relative to an optical or electro-chemical sensor.
Aquaculture - As DO can vary with temperature and salinity, it is important that any measurements of DO are made simultaneously, or with known amounts, of these variables. Where aquaculture occurs in oceans or rivers, DO, temperature and salinity sensors can be connected to a telemetry system for near real-time updates, SMS and email alerts. In ponds or warehouses, where temperature and salinity are more tightly controlled, a portable DO meter or a digital DO sensor can be used.
Scientific research in the lab – Oxygen microsensors are a popular sensor for scientific researchers in the lab. Oxygen microsensors can be used in a wide range of studies including profiling and micro-respiration. A range of low cost, portable DO meters can also be used to check DO levels in tanks and aquaria.
Scientific research in the field – Deep sea sensors can measure DO down to 4000m depth and can be connected to CTD’s, AUV’s and other underwater craft.
why are there so many different units of measurement for dissolved oxygen?
Dissolved oxygen is reported in various, often interchangeable, units of measurement including pO2%, mg/L, ppm and umol mol-1.
pO2% or O2% of air saturation- partial pressure of oxygen or oxygen percentage of air saturation. In a well aerated solution, the percentage of air saturation 100. This is where the partial pressure of oxygen between the solution and the atmosphere is equal. In the atmosphere, oxygen is 20.9% but the solution is said to be saturated so is given a value of 100%.
It is possible for a solution to have dissolved oxygen values greater than 100%. For example, where there is a high amount of photosynthesis and the water body has not come back into equilibrium with the atmosphere. Super-saturation may occur at the bottom of waterfalls or hydroelectric dams where dissolved oxygen can reach over 100%.
mg/L – milligrams per litre is a measure of oxygen content, or concentration, in solution. Dissolved oxygen measurements are widely reported as mg/L however, as discussed above, the concentration value depends on temperature, salinity and barometric pressure. If dissolved oxygen is reported as mg/L, it is always important to check that the value has been corrected for prevailing temperature, salinity and barometric pressure.
ppm – parts per million is equivalent to mg/L and is a measure of dissolved oxygen concentration.
umol/L – micro-molar of oxygen per litre is a unit of measurement commonly used in marine science and oceanography. umol/L factors in the molecular weight of oxygen for concentration measurements.
how to convert between dissolved oxygen units of measurement
Convert from pO2% to mg/L – Temperature, salinity and barometric pressure must be known. Use Henry’s Law to calculate dissolved oxygen from temperature and salinity. Or, use an oxygen solubility chart to find correct dissolved oxygen value.
Dissolved Oxygen (DO) mg/L = (Measured pO2%)*(DO value from oxygen solubility chart)
pO2% = 65, measured in seawater (35 ppt) at 20C
DO mg/L = 0.65 * 7.395 mg/L
DO mg/L = 4.807
Convert mg/L to ppm – 1 mg/L = 1 ppm
Convert mg/L to umol/L - One micromole of oxygen is equal to 0.022391 ml.
DO umol/L = 4.807/0.022391
DO umol/L = 214.685
Therefore, given the above information, in seawater at 20C a dissolved oxygen measurement of 65 pO2% equals 4.807 mg/L or 4.807 ppm or 214.685 umol/L.
Edaphic Scientific’s capabilities
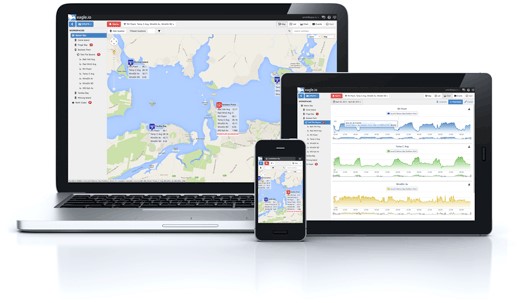 At Edaphic Scientific we want to work with you from the start of your project through to its completion. We can provide:
At Edaphic Scientific we want to work with you from the start of your project through to its completion. We can provide:
- Assistance with project and experimental design
- Procurement of all monitoring equipment, including sensors, data loggers and data management software. Edaphic Scientific is a one-stop shop where we can source and find any necessary equipment for your project from our preferred suppliers or third party suppliers
- Installation and training
- On-going assistance with data interpretation and equipment maintenance
- Data correction and analysis, including statistical analysis with the R-package
- Report and publication preparation including tables, figures, graphs, and manuscript writing
Edaphic Scientific recognises the need for flexible and adaptable sensor and data logging solutions for experimental or environmental monitoring projects.
You can connect dissolved oxygen sensors to our data logging systems, or we can assist you in connecting the sensors to your existing PLC or SCADA system.
 Data can be downloaded directly in the field from data loggers. Alternatively, data can be downloaded over the internet on your iPhone, iPad or desktop computer with the Eagle.io cloud-based, data management software solutions.
Data can be downloaded directly in the field from data loggers. Alternatively, data can be downloaded over the internet on your iPhone, iPad or desktop computer with the Eagle.io cloud-based, data management software solutions.
Edaphic Scientific provides dissolved oxygen sensors with:
Individual loggers or
Whole-System solutions with a centrally located data logger, multiplexers and additional equipment such as dissolved carbon dioxide, hydrogen sulfide, pH electrodes, weather stations and nutrient monitoring.
Data can be collected directly from logging units with a USB download cable or remotely, anywhere in the world, via the mobile phone network and an internet connection.
further reading
http://www.webpages.uidaho.edu/fish503al/002%20Oxygen/2012%20Oxygen%20workshop%20packet.pdf
http://www.fao.org/docrep/field/003/ac183e/ac183e04.htm
http://score.dnr.sc.gov/ktmlpro10/files/uploads/elearning/Understanding_DO.pdf






